Acetone Polar or Nonpolar Solvent
So acetone is more polar than say hexane benzene and chloroform but less polar than acetonitrile acetic acid. Acetone is a polar aprotic solvent.

Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
Other polar solvents include acetone acetonitrile dimethylformamide DMF dimelthylsulfoxide DMSO isopropanol and methanol.
. Acetone BenzeneTolueneChloroform LipidSoluble in Lipids are all insoluble in polar solvents like water but highly soluble in the non-polar or weakly polar organic solvents including ether chloroform benzene and acetone. See answer 1 Best Answer. Ad All of Our Chemicals Are ROV Compliant.
So Is Acetone Polar or Nonpolar. Then again acetone and other carbonyl containing solvents are indeed poor solvents when using strong bases due to. Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane Non-polar Polar.
Can ethanol dissolve lipids. However acetone is still considered a polar aprotic solvent despite the fact that it is relatively acidic and not significantly less acidic than alcohols. As a result the dipole moment of Acetone is around 269 D.
However acetone is still considered a polar aprotic solvent despite the fact that it is relatively acidic and not significantly less acidic than alcohols. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. Polarity of Solvents Author.
Values for relative polarity eluant strength threshold limits and vapor pressure. Acetone has a dipole moment of 288 D and hence is a polar molecule despite possessing characteristics of both the polar and nonpolar substances. Is acetone a nonpolar solvent.
Nonpolar solvents have nonpolar bonds. There are more and less polar solvents. The values in the table below except as noted have been extracted from online and hardbound compilations.
Expertise on Every Level to Craft Science Technology Solutions in Life Science. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Call Us Today to Learn More.
They are composed of atoms that have a negligible difference in electronegativity. Drum Tote Bulk Qty Available - Call 855-TEX-CHEM for details. The values for acetone are µ 288 D and ε 21.
Properties of Organic Solvents. So Is Acetone Polar or Nonpolar. Nonpolar solvents include alkanes pentane hexane and heptane and aromatics.
As a result the dipole moment of Acetone is around 269 D. Polar solvents have polar bonds. It is colorless in appearance and has a.
Acetone is a relatively polar organic solvent. Acetone exists in a liquid state at room temperature. Up to 24 cash back and application.
As a result the dipole moment of Acetone is around 269 D. Then again acetone and other carbonyl containing solvents are indeed poor solvents when using strong bases due to their relatively high acidity. Acetone polar or nonpolar.
Water is a polar solvent. Examples of polar solvents are water acetone acetonitrile dimethylformamide DMF dimethylsulfoxide DMSO. Polar solvents have a positive and a negative charge at different places in their structures and will dissolve other polar substances.
In fact these four solvents are often referred to as lipid-solvents or fat-solvents. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. A protic solvent has an H.
A solvent is polar if it has a dipole moment greater than 16 D and a dielectric constant greater than 5. So Is Acetone Polar or Nonpolar. While larger organic compounds are usually hydrophobic despite having polar groups acetone is small enough to be soluble in water.
So acetone is a polar solvent. Acetone exists in a liquid state at room temperature. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.
Is acetone a nonpolar solvent. Acetone is a polar substance due to the carbonyl group like dissolve like. Acetone exists in a liquid state at room temperature.
Ad Available at Sigma-aldrich.
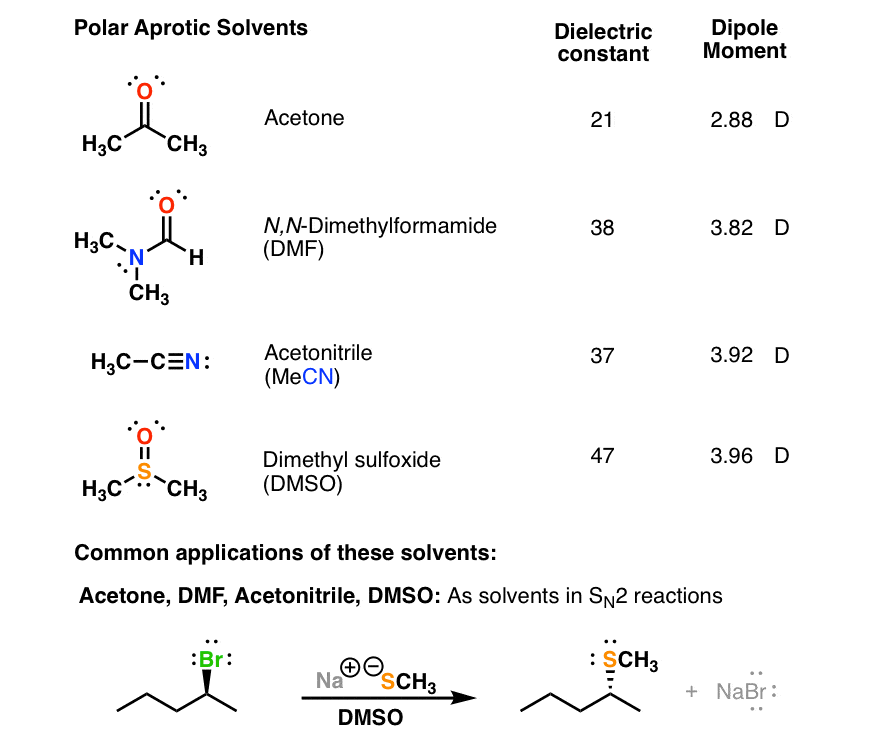
Polar Protic Polar Aprotic Nonpolar All About Solvents
What Makes Acetone A Really Good Solvent What Allows It To Dissolve Both Polar And Non Polar Molecules Quora

Is C3h6o Acetone Polar Or Non Polar Youtube
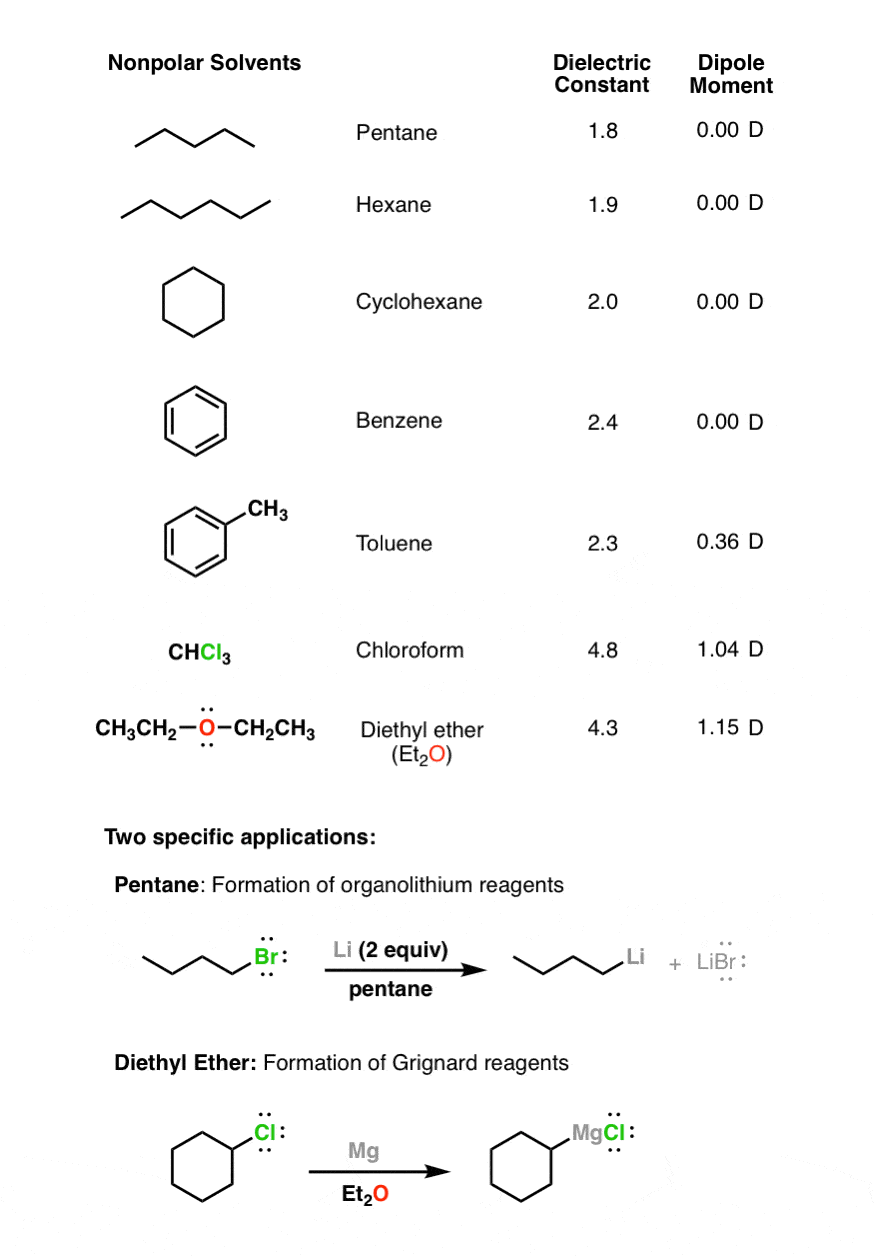
Polar Protic Polar Aprotic Nonpolar All About Solvents
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
0 Response to "Acetone Polar or Nonpolar Solvent"
Post a Comment